Why Are Ionic Compounds Solid at Room Temperature
That is why ionic compounds are solid at room temperature and are hard. In order to melt an ionic compound the strong coulombic forces that act between the ions in the ionic lattice called ionic bonds must be broken.

Why Do Some Solids Dissolve In Water But Others Do Not Why Are Some Substances Gases At Room Temperature But Others Are Intermolecular Force Molecules Force
It is difficult to break the ionic bonds in a compound because of the.

. Energy has to be transferred to a substance in order to melt or boil it. Ionic compounds are solid at room temperature because they have. At room temperature most.
In the case of lithium ion batteries there are two types of electrolytes used in them. Melting and boiling are state changes. Ionic compounds are solids at room temperature.
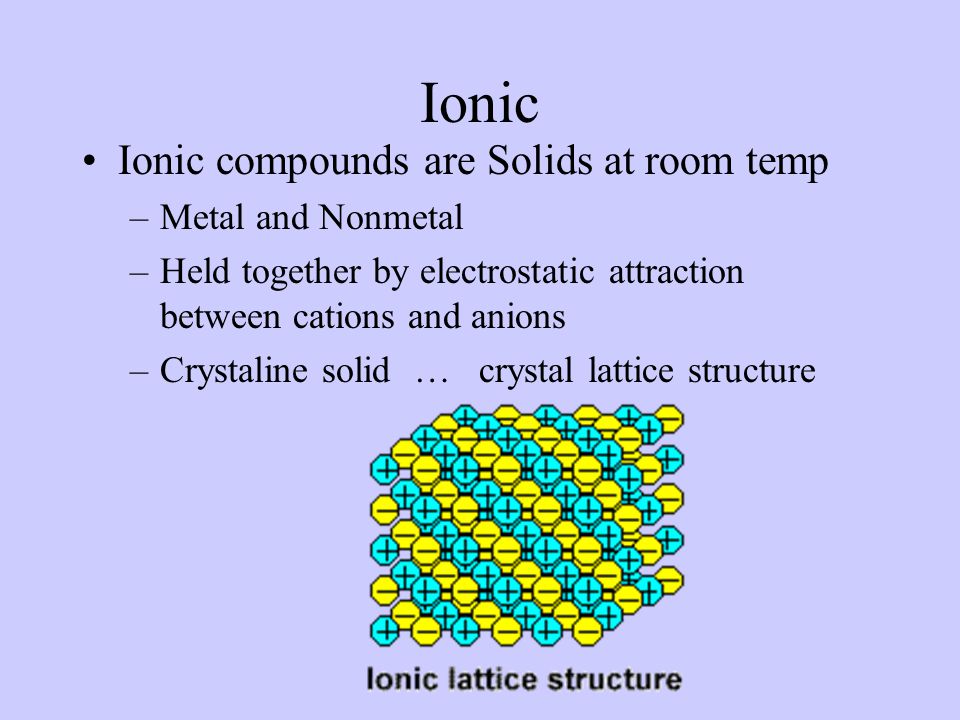
At room temperature ionic compounds tend to be solid covalent compounds are more like to be liquids or gases. Typically they involve ions with relatively complex organic components. 1Ionic compounds are those compounds which are formed by exchange of electrons between oppositely charged ions.
A more mathematical way to look at this if you have empirical values for a compound is to look at the Delta G_textfusion at room temperature. 2As a result there is a stronger bonding between elements than covalent compounds. Crystalline solid at room temperature The force of attraction in an ionic bond is blank.
Solid ionic compounds but molten ones and ones dissolved in aqueous solution are good. 1 These are a result of poor coordination between the ions in solid form. Ionic compounds have high melting and boiling points so they are in the solid state at room temperature.
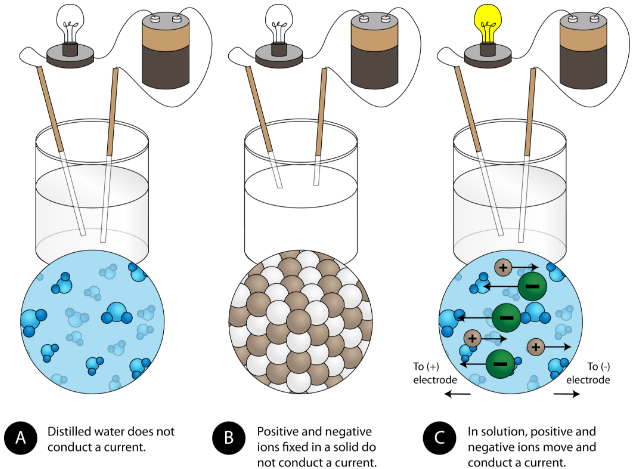
Ionic compounds have a crystal lattice structure are solid at room temperature have high melting points rarely have a smell cannot conduct electricity as a solid but can when dissolved in liquid is soluble in water forms electrolytes in water and are brittle. Delta G_textfusionDelta H_textfusion-TDelta S_textfusion where Delta H_textfusion is negative since it will free up energy in forming the solid and Delta S_textfusion. They are not good conductors of electricity.
This generally requires the input of a lot of energy so the melting points are. Answer 1 of 4. At normal room temperature the strength of an ionic bond is much greater than the individual kinetic energy of the two ions participating in the bond.
Ionic compounds are solid at room temperature and are hard why. All elemental ionic compounds are solid at room temperature however there is a class of room temperature ionic liquids. 4Hence ionic compounds are solid at room temperature.
Hence ionic compounds exist only as solid under normal conditions. Ion with similar charges. 3Due to strong intermolecular forces of attraction the atoms are close.
Because the electrons present in them have a very strong attraction between them. However when the temperature is raised to a certain level the conductivity increases and the electrons are able to move freely in the liquid. Good conductivity between ions.
Ionic compounds are in the solid state at room temperature Why are ionic compounds solid at room temperature. Ionic compounds are not free electrons and conduct electricity in molten state at room temperature. Small distance between the ions.
There are many strong ionic bonds and so large amounts of energy must be transferred to the lattice structure to break these bonds. Why do ionic compounds have high meltingboiling points. Strong attraction between ions.

Properties Of Ionic Compounds 2 2 4 Aqa Gcse Chemistry Combined Science Revision Notes 2018 Save My Exams

Chemical Bonding Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science Science Lessons Chemical Bond Covalent Bonding

Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams

Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams

Ionic Compound Ionic Bonding Crystals

Morgan Gaspard Morganmeaux Instagram Photos And Videos College Notes Solubility Photo And Video

Class 10 Science Metals And Non Metals Notes Chemistry Lessons Science Notes Chemistry Classroom

Pin By Jess Leblanc On School Year Covalent Bonding Chemical Bond Metallic Bonding
Ionic Covalent And Metallic Structures Of Solids Ppt Download

Electron Configurations Ionization Energy Electron Affinity Electron Configuration

Elution Wikipedia The Free Encyclopedia Chromatography For Kids Teaching Chemistry Chemistry Classroom

The Structure Of Ionic Compounds There Are Many Ionic Bonds Electrostatic Forces In An Ionic Compound Such As Sodium Chloride Arranged In Giant Lattice Ppt Download
3 10 Some Properties Of Ionic Compounds Chemistry Libretexts

Room Temperature Ionic Liquid An Overview Sciencedirect Topics

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts




Comments
Post a Comment